REGULATIONS
"We guarantee the regulatory conformity of your products and support you throughout the registration process."
REGULATORY EXPERTISE
We are committed to guiding you through all the various regulatory contexts:
- Medical Devices
- Cosmetics
- Food Supplements
- The conformity of the regulatory documentation
- Notifications to and communications with the relevant authorities
- Listening and support you through your projects
AS THE LEGAL MANUFACTURER, WE ARE RESPONSIBLE FOR THE QUALITY OF THE PRODUCTS WE DELIVER
Product
regulatory files
Export
TECHNICAL DOCUMENTATION
Specific medical device risk / benefit analysis and risk management methodology.
Compilation of usability engineering tests, biocompatibility, clinical and scientific investigation and user experience studies.
1 - MEDICAL DEVICES
European Regulation (EU) 2017/745 relating to medical devices (MD) was adopted in April 2017. These regulations came into force as of 26 May 2021 and our teams worked to ensure our conformity as quickly as possible.
These new regulations have updated the European requirements in terms of boosting safety, post sales monitoring, clinical investigations and traceability in particular. It introduced specifically the UDI, or the Unique Device Identifier, which we have implemented. This enables the distinct and formal identification of all devices on the market.
The devices and their UDIs must be registered on EUDAMED (European Databank on Medical Devices) This database has 6 modules that will gradually be put online. We have already registered with the first module: “Registering Economic Operators” and been given our SRN (Single Resgistration Number) code.
In addition, in compliance with the Regulations, we have appointed within the company a Person Responsible for Regulatory Compliance (PRRC).
Furthermore, as part of the placing on the market process for our medical devices, we compile:
- A biological evaluation in accordance with the ISO 10993 Standard
- A clinical evaluation
- A risk management file including a risk / benefit analysis in accordance with the ISO 14971 Standard
- Usability engineering tests recorded in the usability dossier in accordance with the IEC 62366-1 Standard
- A packaging conformity check for all our customers
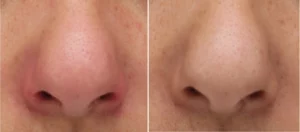
We manufacture Class I and IIa medical devices: Bag-on-valve sprays, pump sprays, baby aspirator, etc.
If the medical device is Class I, the regulation allows self-certification.
For our Class IIa products, an evaluation request must be submitted to our Notified Body (NB). Following the evaluation and approval of the notified body, the products are granted their CE Certificates and CE Markings.
Once the declaration of conformity has been issued or the CE Certificate obtained, we then take responsibility for declaring the placing on the market of the product with the National Medecines and Healthcare Product Safety Agency (ANSM).
2 - COSMETICS
Cosmetic products are substances or combinations intended to be in contact with the surface areas of the human body or with teeth and mouth mucosa to, exclusively or primarily, clean, perfume, modify the appearance, protect, maintain in good condition or remedy bodily odours. These products are governed by Regulation (CE) 1223/2009.
In the context of these Regulations, we are responsible for:
- The cosmetics status
- The regulatory validation of the formulas
- The compiling of the Product Information Sheet (PIS)
- The verification of the conformity of the packs for all our customers
- The completion of Section B of the cosmetic product safety evaluation report, by a toxicologist, in accordance with Article 10 and Appendix I of the Regulations
- The monitoring of tests (efficacity, application, instrumental measurements) to evidence the claims made for our products
Before placing on the market, we declare our products on the CPNP (Cosmetic Products Notification Portal).
3 - FOOD SUPPLEMENTS
A food supplement is a food product that is intended to supplement normal dietary regimes and that offers a concentrated source of nutrients or other substances that have nutritional or physiological effects. These types of products are governed by Directive 2002/46/CE and Regulation (EU) 1169/2011. Additional legislative requirements are also applicable in France (Plant Decree, Substance Decree).
In the context of the regulations relating to food supplements, we compile:
- The Regulatory file
- The food supplement status
- The regulatory validation of the ingredients, their concentrations and the Reference Nutritional Values (RNV)
- The verification of the conformity of the packagings for all our customers
Prior to placing on the market, we submit declarations for our products to the Competition, Consumer Affairs and Anti-Fraud Agency (DGCCRF in France). In compliance with Articles 15 and 16, the DGCCRF makes our declarations accessible to all companies located in the European Union or in another State within the European Economic Space (Iceland, Norway, Lichtenstein). These files are available on the Teleicare platform.
4 - ALL OUR PRODUCTS
YSLAB implements:
- Proactive and reactive materiovigilance, cosmetovigilance and nutrivigilance processes. Within the company we have a Local vigilance manager who has been registered with the ANSM.
- Active regulatory and standard watch processes.
Finally, for all of our products, we carry out regular Post-Market Surveillance (PMS) and produce a Periodic safety update report (PSUR) in particular for medical devices.

EXPORT OPERATIONS
We support you throughout the registration process for your products and even after the placing on the market.
The export related operations include:
– Monitoring product registration documentation
– Strategic advice in terms of product registrations
– International Regulatory and standards watch
– Transfer and communication of all documents required by the relevant authorities
We also provide support through the various administrative procedures:
- Interpretation assistance
- Drawing up registration/renewal files
Joint approval of labelling/marketing tools in accordance with the applicable regulations
- Document authentication procedures (Notarisation, apostille, legalisation, etc.)
- Regulatory Watch services
USE OUR SERVICES
Contact us!