RESEARCH &
DEVELOPMENT
"Our research and development work provides the foundations for our innovations and underpins the effectiveness of our products."

THE SEA, A SOURCE OF GREAT HEALTH
It is our belief that the sea is our natural ally in good health because of its inestimable beneficial qualities. Naturally rich in minerals and trace elements, the sea is a fundamental element thus constituting a unique product range. Our expertise has been built around these key fundamentals.
Our research teams have proven the effectiveness of marine actives and the formulas developed around these. They also make sure there is no risk of toxicity.
FORMULATION
Our teams work on innovative formulas adapted to match consumer expectations in terms of efficacy and safety. YSLAB is focused on research into marine ingredients for inclusion into its formulas.
The dosage form – Pump spray, bag-on-valve spray, gel, cream, capsules, tablets, powder, etc. – ensures a targeted and effective result with maximum ease of use. This choice makes sure the chosen actives reach the target area of the body.
YSLAB may decide to protect its innovations with patents.



ANALYTICAL CONTROL
In our laboratory, our teams support the development of new products by characterizing the raw materials and identifying the specific markers. Analytical checks are performed throughout the product lifecycle with the carrying out of release analyses and the monitoring of stability. We work jointly with microbiology and physical-chemical technical support centres to ensure we have control over the characterisation of the products.
- Characterisation of ingredients, selection of specific analytical markers.
- Development of analytical methods.
- Analytical checks on raw materials and finished products.
- Validation of analytical methods.
- Stress testing during the formulation process.
- Stability studies.
- Specialised in marine actives and in particular those in the seawater matrix.
EXPERTISE IN CELLULAR ANALYSIS
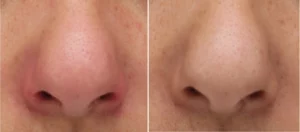
Our expertise in cellular testing and the application of cellular models enables us to evidence our claims by evaluating the protective effects and the side-effects (toxicology).
- ENT applications (respiratory epithelial cells, mucosa epithelial cell model)
- Oral applications (gingival epithelial cells)
- Ophthalmic applications (corneal cell lines, retina cell lines)
- Fluorescence: Quantitative analysis (cytometry), Qualitative analysis (microscopy)
- Luminescence
- Absorbance quantification
Examples of in-vitro studies:
- Inflammation, oxidative stress, wound healing, cellular degeneration or death communication channels…
Our work is peer-reviewed by the scientific community as we publish our results in specialised journals around the world, as well as through our presentations to international conferences.


CLINICAL EVALUATION
The efficacy and tolerance of our solutions are regularly evaluated by experts.
New proofs are established and published in collaboration with medical experts and pharmacists. Clinical investigations, user experience studies, and data collected from the literature ensure the compliance to an optimal benefit/risk ratio.
DEVELOPMENT
From the formulation to the means of administration, our Development department is responsible for designing effective, safe and appropriate medical devices, cosmetics and food supplements. This starts with the definition of the specifications for the raw materials and long-term access to the resources to avoid supply chain breakages.
Specifications for the finished product and the packaging are also drawn up. An evaluation of the raw materials is carried out, together with a technical toxicity analysis.
In terms of manufacturing, we compile a pre-stability study and a specifications sheet. Liaising with the Quality department, we examine and select the best service provisions.
With the Marketing team, we work on the production of supports, labelling and containers. The team is also responsible for drawing up the regulatory technical, quality and customer documentation.
Our developments are validated during the industrial transition.
AN ORGANISATION CONNECTED TO ITS LOCAL ROOTS
The BlueHuman project is focused on the economic potential of marine bioresources for biomaterial applications. The project brings together a consortium of European academic and SME research teams.
The HealthSea project aims to drive forward scientific research into seawater. The project has been recognised for its innovative character as part of France’s “Investissements d’Avenir program” (investment programme for the future).
We are also part of the Blue Train consortium which is working to position the Brittany region as an excellence hub for vocational training in the marine biotechnologies field.
USE OUR SERVICES
Contact us!